|
by Ashley Jordan Ferira, PhD, RDN
A food fortification trial demonstrated that 600 IU of daily vitamin D3 had a significantly greater impact than 600 IU of daily vitamin D2 in elevating serum blood levels of 25-hydroxyvitamin D [25(OH)D].1-2 Vitamin D is essential for skeletal health and many emerging extraskeletal physiological processes, but remains one of the most common micronutrient dietary gaps, resulting in widespread hypovitaminosis D globally. Understanding how much vitamin D the body needs daily, in what form, and from what sources is still being discovered. There are two forms of vitamin D: plant-based ergocalciferol (vitamin D2) and animal-based cholecalciferol (vitamin D3). D2 can be found in UV-irradiated mushrooms, certain fortified foods (breakfast cereals, margarine, and milk), dietary supplements, and vitamin D prescription medications. D3 is found in oily fish, egg yolks, fortified milk, and dietary supplements.4 Chemically, D2 and D3 are almost identical except for key side chain differences, with D2 having an additional double bond. D3 has been shown to have a higher affinity to the vitamin D binding protein, hepatic 25-hydroxylase (enzyme that converts vitamin D to the circulating 25(OH)D form) and vitamin D receptor. Whether these chemical and cellular differences translate into differential abilities in raising serum 25(OH)D, the clinical measure of vitamin D status, has been a hotly debated topic since the early 20th century.5 Research literature to date demonstrates a robust case gaining momentum for vitamin D3 and against vitamin D2 for supplementation.4-5 In particular, a 2012 systematic review and meta-analysis by of randomized controlled vitamin D supplementation trials in humans explored a head-to-head comparison of vitamin D2 vs. D3 in raising serum 25(OH)D; vitamin D3 was clearly shown to be more efficacious at raising and maintaining serum 25(OH)D levels than vitamin D2.4 Authors concluded that vitamin D3 may be considered the preferred choice for supplementation.4 Since natural sources of vitamin D (dietary input and UVB exposure from the sun) are limited, and a daily vitamin D supplementation regimen is a personal health decision, vitamin D fortification of the food supply is an important, strategic public health measure to help increase dietary vitamin D intake and improve status in the general population.6 Clarity is needed to elucidate whether D2 and D3 are equally effective sources for food fortification, since both forms are currently utilized in the food supply.6 A study by Tripkovic et al. helps to shed light on key differences.1 Results were published in The American Journal of Clinical Nutrition by Dr. Laura Tripkovic and colleagues from a randomized, double-blind, placebo-controlled food fortification trial that included 335 healthy South Asian and white European women aged 20–64 years.1 Participants were randomized to one of five groups: 1) Placebo: Placebo juice with placebo biscuit 2) D2J: Juice supplemented with 15 mcg vitamin D2 with placebo biscuit 3) D2B: Placebo juice with biscuit supplemented with 15 mcg vitamin D2 4) D3J: Juice supplemented with 15 mcg vitamin D3 with placebo biscuit 5) D3B: Placebo juice with biscuit supplemented with 15 mcg vitamin D3 Fifteen mcg of vitamin D is equivalent to 600 IU of vitamin D, which is the US Recommended Daily Allowance (RDA) for ages 1-70 years.7 The daily food-fortified intervention was 12 weeks long during the winter, and serum total 25(OH)D levels were collected at baseline, week 6 and week 12. Data analysis combined ethnic groups. D3-fortified consumption was shown to be twice as effective as D2 in raising 25(OH)D serum levels in the body.1 While the placebo group experienced a 25% reduction in serum 25(OH)D levels over the course of the study, the D2J and D2B groups saw 25(OH)D increases of 33% and 34%, respectively. Most effective, however, were the D3 groups, with 25(OH)D increases in the D3J and D3B groups of 75% and 74%, respectively. The D3J group induced higher incremental increases in 25(OH)D levels: 16.9 nmol/L higher than the D2J group, 16.0 nmol/L higher than the D2B group, and 42.9 nmol/L higher than the placebo group.1 Both juice- and biscuit-supplemented vitamin D3 groups demonstrated similar results, with no statistical differences seen between D3J and D3B groups.1 Compared to white European women, the South Asian women demonstrated a greater increase in 25(OH)D levels in response to both D2 and D3, which was likely caused by their lower baseline vitamin D status.1 This study shows that modest supplementation levels (600 IU daily) of D3 in food and beverage sources twice as effective at raising serum levels of 25(OH)D than vitamin D2.1This study and previous supplementation studies may impact future policy and practice for vitamin D supplementation source. Additional research addressing dose response, bioactivity of D3 versus D2 and the impact of foods with high levels of vitamin D3 is needed.2 Why is this Clinically Relevant?
Link to abstract Citations
0 Comments
The female-centric 411 on this essential nutrientby Ashley Jordan Ferira, PhD, RDN
Overview Vitamin D research and daily news headlines are ubiquitous. PubMed’s search engine contains over 81,800 articles pertaining to vitamin D.1 Information abounds on vitamin D, but the vetting and translation of that information into pragmatic recommendations is harder to find. Evidence-based takeaways and female-centric recommendations are crucial for healthcare practitioners (HCPs), their female patients and consumers alike. Women are busy, multi-tasking pros, so practical, personalized takeaways are always appreciated. In other words, women need the “411” on vitamin D. Merriam-Webster defines “411” as “relevant information” or the “skinny”.2 So for all of you busy women, here’s the skinny on vitamin D. Let’s explore common questions about this popular micronutrient. Q: Is vitamin D more important for younger or older women? A: All of the above. Vitamin D plays a critical role in women’s health across all life stages, from fertility/conception, to in utero, childhood, adolescence, adulthood, older adulthood, and even in palliative care. Vitamin D is converted by the liver and kidneys into its active hormone form: 1,25-dihydroxyvitamin D. This dynamic hormone binds nuclear receptors in many different organs in order to modulate gene expression related to many crucial health areas across the lifecycle, including bone, muscle, immune, cardiometabolic, brain, and pregnancy to name a few.3 Q: I am a grandmother. Are my vitamin D needs different than my daughter and granddaughter? A: Yes, age-specific vitamin D recommendations exist. As an essential fat-soluble vitamin, women need to achieve adequate levels of vitamin D daily. Age-specific Recommended Dietary Allowances (RDA) from The Institute of Medicine (IOM),4 as well as newer clinical guidelines from The Endocrine Society,5 provide helpful clinical direction for daily vitamin D intake and/or supplementation goals. The IOM RDAs4 are considered by many vitamin D researchers to be a conservative, minimum daily vitamin D intake estimate to support the bone health of a healthy population (i.e. prevent the manifestation of frank vitamin D deficiency as bone softening: rickets and osteomalacia): Infants (0-1 year): 400 IU/day Children & Adolescents (1-18 years): 600 IU/day Adults (19-70 years): 600 IU/day Older Adults (>70 years): 800 IU/day The Endocrine Society’s clinical practice guidelines5 recommend higher daily vitamin D levels than the IOM, with a different end-goal: raising the serum biomarker for vitamin D status [serum 25-hydroxvitamin D: 25(OH)D] into the sufficient range (≥ 30 ng/ml) in the individual patient: Infants (0-1 year): At least 1,000 IU/day Children & Adolescents (1-18 years): At least 1,000 IU/day Adults (19+ years): At least 1,500 – 2,000 IU/day Q: I am a health-conscious woman who eats a nutritious, well-rounded diet. I should not need a vitamin D supplement, right? A: Not so fast. Daily micronutrient needs can be met via diet alone for many vitamins and minerals. Vitamin D is one of the exceptions, which is why an alarming number of Americans (93%) are failing to consume the recommended levels from their diet alone.6-7 Very few foods are endogenous sources of animal-derived vitamin D3 (cholecalciferol) or plant-derived vitamin D2 (ergocalciferol). Some natural vitamin D sources include certain fatty fish (e.g. salmon, mackerel, sardines, cod, halibut, and tuna), fish liver oils, eggs (yolk) and certain species of UV-irradiated mushrooms.8 In the early 20th century, the US began fortifying dairy and cereals with vitamin D to help combat rickets, which was widespread. For example, one cup (8 fluid ounces) of fortified milk will contain approximately 100 IU of vitamin D. Even though some food sources do exist, the amounts of these foods or beverages that an adult would need to consume daily in order to achieve healthy 25(OH)D levels (> 30 ng/ml) is quite unrealistic and even comical to consider. For example, you would need to toss back 20 glasses of milk daily or 50 eggs/day to achieve 2,000 IU of vitamin D! In contrast, daily vitamin D supplementation provides an easy and economical solution to consistently achieve 2,000 IU and any other specifically targeted levels. Q: I enjoy the outdoors and get out in the sun daily, so I should be getting all of the vitamin D that I need, correct? A: Vitamin D is a highly unique micronutrient due to its ability to be synthesized by our skin following sufficient ultraviolet (UV) B irradiation from the sun. Many factors can result in variable UV radiation exposure, including season, latitude, time of day, length of day, cloud cover, smog, skin’s melanin content, and sunscreen use. Furthermore, medical consensus advises limiting sun exposure due to its established carcinogenic effects. Interestingly, even when dietary and sun exposure are both considered, conservative estimates approximate that 1/3 of the US population still remains vitamin D insufficient or deficient.9 Q: What factors can increase my risk for being vitamin D deficient? Are there female-specific risk factors? A: Although the cutoff levels for vitamin D sufficiency vs. deficiency are still debated amongst vitamin D researchers and clinicians, insufficiency is considered a 25(OH)D of 21-29 ng/ml, while deficiency is < 20 ng/ml.5 Therefore, hypovitaminosis D (insufficiency and deficiency, collectively) occurs when a patient’s serum 25(OH)D falls below 30 ng/ml. The goal is 30 ng/ml or higher. Ideally, vitamin D intake recommendations4-5 and therapy are personalized by the HCP based on patient-specific information, such as baseline vitamin D status, vitamin D receptor single nucleotide polymorphisms and other pertinent risk factors. Common risk factors for vitamin D deficiency to look out for include: -> Overweight/obesity -> Older age -> Regular sunscreen use -> Winter season -> Frequent TV viewing -> Dairy product exclusion -> Darker skin (more melanin) -> Not using vitamin D supplements -> Malabsorption disorders (e.g. bariatric surgery, IBD, cystic fibrosis) -> Liver disease -> Renal insufficiency -> Certain drug classes: weight loss, fat substitutes, bile sequestrants, anti-convulsants, anti-retrovirals, anti-tuberculosis, anti-fungals, glucocorticoids -> Lastly, additional female-specific risk factors to look out for include exclusive breastfeeding while mother is vitamin D insufficient (can result in infant being vitamin D deficient) and certain cultural clothing that covers significant amounts of skin surface area (e.g. hijab, niqab). Key Takeaways
Food first, but fill the gap: The case for vitamin D supplementationAshley Jordan Ferira, PhD, RDN
If you can have a favorite nutrient, mine would be vitamin D. Historically famous for its essential, classical role in calcium and phosphorus homeostasis and bone physiology (think rickets prevention), the past few decades of research have unveiled diverse, extraskeletal health roles for vitamin D, including but not limited to the immune system, cardiometabolic pathophysiology, cancer, pregnancy, etc. Whether consuming vitamin D2 or D3 (FYI, the latter more potently impacts vitamin D status),1 vitamin D ultimately circulates in the 25-hydroxyvitamin D [25(OH)D] form (the clinical biomarker used to measure vitamin D status) and acts throughout the body as a pleiotropic hormone in its active form, 1,25-dihydroxyvitamin D [1,25(OH)2D]. Unlike other nutrients, this fat-soluble vitamin is obtainable via several unique routes: the skin with adequate UVB exposure, a handful of natural food sources, a few fortified foods, dietary supplements, and even prescription drugs. The problem is that few foods naturally contain vitamin D (e.g., egg yolk, certain fatty fish, fish liver oil, and certain species of UV-irradiated mushrooms), and fortified foods offer relatively small amounts (e.g., 100 IU vitamin D per 8 oz cup of fortified milk or orange juice),2 so vitamin D supplementation becomes a strategic solution. In the eloquent words of pediatrician and vitamin D researcher, Carol Wagner, MD: “Something so simple- vitamin D supplementation- could improve the health status of millions and so becomes an elegant solution to many of our health problems today.” If it’s possible to be defensive of a micronutrient, I am protective of vitamin D. Non-evidence-based rumors and negative media attention targeting vitamin D are common. Some of the misinformation is hype from anti-supplement camps who make broad, sweeping statements that lack scientific substantiation. But not all of the vitamin D myths originate from bias or lack of intellectual rigor. After all, who has time to keep up with the impressive, daily output of new vitamin D research? Clinicians certainly do not have the luxury of time, not in the current healthcare paradigm. Nevertheless, when inaccurate conclusions are propagated to patients about vitamin D and their health, that’s more harm than good. So, let me help out. This blog series explores some of the most common vitamin D myths. Let’s tackle just 1 myth today: Myth: I get enough vitamin D from food, so I don’t need a vitamin D supplement. Can you meet your vitamin D needs from food alone? Well, that depends on how you define “needs.” Let’s talk about the 2 major (and quite different) sets of vitamin D recommendations. First, the National Academy of Medicine (NAM), formerly known as the Institute of Medicine, provided vitamin D Recommended Dietary Allowances (RDAs) in 2010.3 Here’s how much vitamin D NAM says that we (Americans and Canadians) need based on bone health research (think rickets and osteomalacia prevention, calcium absorption, etc.):3
But I have a bone to pick (pun intended) with NAM’s vitamin D recommendations. I find them to be problematic, if not contradictory at times, for a few key reasons. To start with, the RDA is by definition “the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97-98%) healthy people.”4 Well, that misses the unhealthy people. Since 2/3rd of the country are overweight or obese5 and heart disease and cancer are the #1 and #2 causes of mortality in the US, respectively,6 one can extrapolate that the vitamin D RDAs do not apply to a decent chunk of the gen pop. In fact, research indicates that overweight and obese individuals require more vitamin D than their lean counterparts,7 but the NAM recommendations fail to consider adiposity. Second, lumping a toddler and 68-year-old grandmother in the same RDA category (i.e., ages 1-70 years) seems to lack nuance. Skeletal health is critical throughout life, but you cannot tell me that the vitamin D needs for the rapidly accruing skeleton in childhood and adolescence are no different than an adult or older adult’s skeletal needs. Third, the RDAs for daily vitamin D intake are simply incongruent with the serum 25(OH)D cutoffs NAM also published in 2010. They provided the following 25(OH)D ranges:
In the sage words of the late Bob Heaney, MD: “We’ve been able to show that the (vitamin D) RDA barely budges the blood 25-hydroxyvitamin D level.”8 Thanks to Dr. Heaney, who made invaluable research contributions to the field of vitamin D, we know that 100 IU/day of vitamin D increases serum 25(OH)D concentrations by approximately 1 ng/mL.9 That means that 1,000 IU/day of vitamin D would raise 25(OH)D by about 10 ng/mL. Although weight status, age, and the patient’s baseline vitamin D status can variably impact the supplementation response, this “rule of thumb” can be used to roughly calculate vitamin D supplementation needs. For example, let’s take a patient: me. I have limited UVB sun exposure and consume some foods that contain vitamin D (e.g., milk, eggs, salmon) but irregularly. My daily 5,000 IU vitamin D3 supplement has my serum 25(OH)D at 54 ng/mL. It stays between 50-60 ng/mL, which is in the sufficient range. But I’m an anomaly. Nationally representative research backs up the fact that Americans are not getting adequate vitamin D from their diets.10-11 First, 93% of Americans 2 years and older are failing to consume at least 400 IU/day of vitamin D from diet alone, and this estimate includes fortified food sources.10 Even when diet plus sun exposure are both thrown into the mix, about 1/3rd of the US population has serum 25(OH)D levels associated with vitamin D insufficiency or deficiency.11 For more details on vitamin D deficiency and why it persists, check out this blog. Dietitians (I am one) and other clinicians love to preach “food first.” That slogan is true but ignores research on key nutrient gaps. I prefer to say, “food first, then fill the gaps.” And in the case of vitamin D, the gap is practically guaranteed, except for the outlier patient who’s knocking back fish liver oils and irradiated mushrooms. Lastly, a more current and scientifically and clinically nuanced set of guidelines exist. One year after the NAM recommendations were released, several of the world’s leading vitamin D researchers convened to review the evidence to date, resulting in the 2011 publication: Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline.12 The US Endocrine Society’s conclusions are harmonious with the mindset of a clinician, who is tasked with addressing the vitamin D needs of unique patients. The guideline recommends higher daily vitamin D levels than NAM, with a different and logical purpose in mind: Raising serum 25(OH)D levels into the sufficient range (≥ 30 ng/ml):12
Individual genetic differences for the vitamin D receptor (VDR) (i.e., gene polymorphisms like Cdx2, Apa1, Fok1, Taq1) are another important facet to weave into each patient’s unique vitamin D story, underscoring the prudence of a personalized lifestyle medicine approach to treat the individual. Takeaway: No, you cannot satisfy your vitamin D needs from food alone. If you plan to raise and maintain your serum 25(OH)D level (the biomarker that indicates vitamin D status) in the sufficient range for skeletal and extraskeletal health, that will require daily vitamin D supplementation. Remember, 30 ng/mL is not the goal. It’s the cutoff for insufficiency. Here’s a sneak peak at some of the additional vitamin D myths that will be covered in future blogs:
Citations
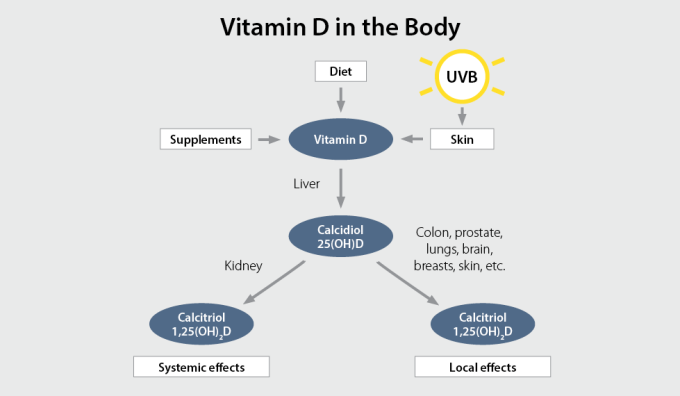
Did you know that getting just 10 minutes of sunshine (ultraviolet B, or UVB) per day helps the body create approximately 10,000 IU of vitamin D?1 This nutrient is necessary for the health of your bones, as well as overall health.2 However, during the months of November through February, and if you live north of Atlanta, there won’t be enough UVB rays to penetrate through the atmosphere and help your skin generate this vital nutrient. So is there something you can do? Sometimes you just have to create your own sunshine. And considering that three-quarters of teens and adults in the United States are deficient in vitamin D,3 as well as 1 billion people worldwide,4 this is where supplemental vitamin D can really help. Natural dietary sources of vitamin D are few (e.g. fatty fish, eggs), and fortified dietary sources such as milk, orange juice and cereal provide minimal amounts of vitamin D. This is why vitamin D is one of the most common nutrient gaps and also one of the easiest to address via supplementation. Why is vitamin D important? Vitamin D is a fat-soluble vitamin that regulates bone growth and mineralization and plays an important role in ensuring the muscles, heart, lungs, and brain function properly.2 Vitamin D has also been shown to support immune function. Vitamin D is not only an essential vitamin but also acts as a hormone in the body. Vitamin D that you obtain from the sun, food, beverage, or supplements must be first activated by the liver which converts the vitamin D to 25-hydroxyvitamin D (25(OH)D), also known as calcidiol.2 It is then converted by the kidneys and target tissues in the body to the biologically active form 1,25-dihydroxyvitamin D (1,25(OH)2D), also known as calcitriol.2 Calcitriol is the active, hormone form, which supports a variety of physiological functions, including helping the body regulate levels of calcium and phosphorus, as well as mineralize bone.5 Vitamin D deficiency What does it mean to be deficient in vitamin D? Measuring serum concentrations of 25(OH)D rather than 1,25(OH)2D is a better indicator of vitamin D status in the body due to its longer half life. Certain groups define vitamin D deficiency as 25(OH)D level less than 20 ng/mL (50 nmol/L).6 Vitamin D deficiency can be an issue for many people, including:7
Finding out if you need more vitamin D Measuring your vitamin D levels via a blood test is the only way to definitively know if you’re getting enough of this nutrient. With a 25(OH)D blood test from your healthcare practitioner, you will know your vitamin D levels and whether you need to take a supplement. Optimal levels of vitamin D vary according to different scientific organizations. For example, for adults the Vitamin D Council recommends daily supplementation with 5,000 IU of vitamin D3 when you cannot get enough sun to achieve a status between 40-60 ng/ml; whereas, the Endocrine Society recommends 1500-2000 IU/day.7-8 Higher levels are recommended to address deficiency.8 It is also important to recheck vitamin D levels two months after beginning a supplement regimen, and adjust as needed based on your practitioner’s recommendations. Which D is right for me? It’s important to get the form of vitamin D that is most bioavailable to the body. There are two kinds of vitamin D—D2 and D3. Vitamin D2 (ergocalciferol), is found in plants such as lichens and mushrooms, which are often irradiated by growers to boost nutritional value. Some soy and almond milks are also fortified with vitamin D2. Vitamin D3 (cholecalciferol) is the natural form of this nutrient that is created by the body with sun exposure, and research has shown that the D3 form increases the total circulating level of 25(OH)D significantly more effectively than D2.9-10 Vitamin D3 is found in small amounts in oily fish such as cod and salmon, egg yolks, as well as fortified cereals and milk, and some commercial mushrooms. Additionally, vitamin D3 has been shown to maintain adequate amounts of serum vitamin D levels during the winter months.11 There are also special sunlamps to help the skin generate vitamin D, but because of the risk for skin damage from ultraviolet rays, many healthcare practitioners don’t recommend using them. Make D your favourite letter for better health Whether you’re lucky enough to get the vitamin D you need from the sun all year around, or taking a vitamin D supplement, you’re wise to ensure you get enough of this vital nutrient. If you’re wondering whether you need more vitamin D, ask your healthcare practitioner. References:
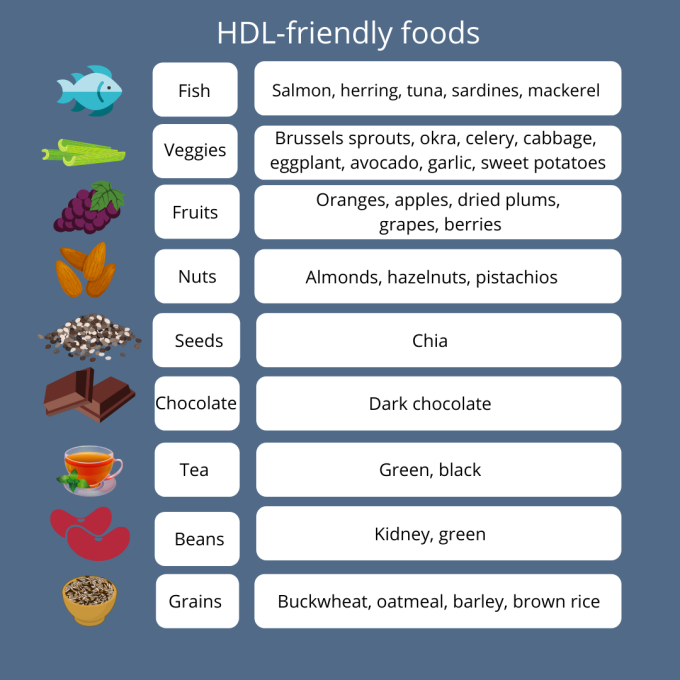
High-density lipoproteins (HDL) are also known as “good” cholesterol. These particles help to remove cholesterol from the body by carrying it from the rest of the body to the liver, where it’s eliminated. There are a number of ways to help keep your HDL levels in the optimal zone, including exercising, quitting smoking, and taking supplements. There are also certain foods that, in correlation with exercise, can help to raise your HDL levels (and/or improve the ratio of your HDL to LDL levels). What do these foods have in common? High in healthy fats: Foods such as fatty fish, nuts, and seeds are great dietary sources of omega-3 fatty acids. Omega-3s are known for their heart-healthy benefits, but they also have the power to raise HDL levels.3,4 High in antioxidants: Known for the role they play in supporting a healthy immune system, antioxidants help to protect our bodies from the damage caused by oxidative stress. Foods high in antioxidants such as berries, grapes, and green tea have an added benefit: A higher antioxidant level is associated with improved HDL numbers.5 HDL is actually an antioxidant itself, helping to prevent the oxidation of lipids on LDL particles and helping to remove free radicals.6,7 So which foods should you add to your next grocery list? Conclusion
So if your healthcare practitioner has suggested you increase your HDL levels to help optimize your health, consider adding one or more of these foods to your daily diet! References: 1. Zhou Q et al. Int J Environ Res Public Health. 2015;12(5):4726-4738. 2. Brown L et al. Am J Clin Nutr. 1999;69(1):30-42. 3. Franceschini G et al. Metabolism. 1991;40(12):1283-1286. 4. Yanai H et al. J Clin Med Res. 2018;10(4):281-289. 5. Kim K et al. Nutrients. 2016;8(1):15. 6. Xepapadaki E et al.. Angiology. 2020;71(2): 112-121. 7. Brites F et al. BBA Clinical. 2017;8:66-77. By Melissa Blake, ND
Modern-day living has granted us access to an abundance of both food and artificial light, contributing to an endless opportunity for food production and consumption. The opportunity for overindulgence can lead to a number of health issues, including changes in sleep, mood, and energy, unwanted weight gain, heart conditions, memory issues, and digestive complaints. The circadian diet offers a solution to these negative outcomes and is basically a way of aligning intermittent fasting with your circadian rhythm that takes into account not only what you eat, but when you eat. What is the circadian diet? While similar to intermittent fasting, the circadian diet divides a 24-hour day into two windows—one window when food is consumed and the other spent fasting (no food, only water). The circadian diet emphasizes eating in sync with the body’s natural tendencies and instincts. This means eating during daylight hours during a window that is approximately 12 hours long and falls somewhere between sunrise and sunset, with evidence suggesting the earlier the eating window closes, the better.1 Eating in this way, with an emphasis on dietary timing, can help support the body’s natural circadian rhythm and contribute to overall health and wellness.1 The benefits of the circadian diet The circadian diet, in part due to a positive impact on circadian rhythm, contributes to substantial benefits on sleep and metabolic health. This often means better energy, fewer cravings, blood sugar control, improved digestion, and weight loss.1 When done properly, the circadian diet is a safe and effective way of eating, and modifications can be made to suit almost any lifestyle, cuisine, or dietary preferences. A sample day on the circadian diet Morning routine: A day on the circadian diet involves more than just food. It starts with waking at a consistent time, ideally with the sun. After you wake, drink water or herbal tea to help meet your hydration needs. Follow that up with some gentle stretching or movement to help get the blood flowing and energize the body. Try to get outside within one hour of waking in order to help optimize your body’s internal clock. Break-the-fast: Next, begin the 12-hour eating window within two hours of waking by literally breaking the fast with a well-balanced meal that includes protein, fruit, vegetables, and good-quality fat. Aim to consume most of your calories earlier in the day, with a smaller meal to close the eating window. Ideally, you’ll want to stop eating two or more hours before bedtime in order to allow the body sufficient time for digestion. “Aim to consume most of your calories earlier in the day, with a smaller meal to close the eating window. Ideally, you’ll want to stop eating two or more hours before bedtime in order to allow the body sufficient time for digestion.” Close the window: During the fasting window, which ideally starts early in the evening, drink water and herbal tea to stay hydrated. Consider a gentle movement routine and other calming activities to promote sleep, such as journaling, light reading, and breathing practices. Another important aspect of the circadian health diet involves limiting technology, especially during the evening hours. Shut off all screens and try to reduce your exposure to light for at least one hour before bed to reduce any potential disruptions to sleep patterns.2 Finally, ensure you have a dark, cool, and comfortable sleep space combined with a consistent bedtime to help round out your day. Things to consider with the circadian diet Initially, people may experience hunger during the fasting period of the circadian diet, especially those who struggle with nighttime eating or blood sugar control. These issues generally can be avoided if the fasting period is introduced slowly over a period of time, with gradual increases to the fasting window. Many people have predetermined thoughts about food and it can be helpful to clean that slate and unlearn what you think you know about food. For example, avoid categorizing food as “good” vs. “bad” or even “breakfast” vs. “supper” foods. Aim to become more adventurous in the kitchen – this could mean eating chicken and salad for breakfast or a hearty bowl of lentil soup. Following a circadian diet means listening to your body and its needs. This can be a challenge if you struggle with unhealthy food cravings or are new to a whole foods way of eating. Ask for help and take it slow. If you have a significant medical concern or if you’re unsure where to start, consider speaking with a knowledgeable healthcare provider who can help support you with a personalized plan. Every step, even if it seems small, counts. The key is what you choose to do every day. Daily habits and routines have the most significant impact on circadian rhythm and offer an opportunity for anyone to optimize circadian health, contributing to better overall health and wellness. References: 1. Zheng D et al. Trends Immunol. 2020;41(6):512-530. 2. Aulsebrook AE et al. J Exp Zool A Ecol Integr Physiol. 2018;329(8-9):409-418. by Bianca Garilli, ND
When doctors measure our cholesterol, they look at the vehicles that carry it in our blood—which gives them a clue what our body is doing with that cholesterol. The two most common and well-known vehicles are low-density lipoproteins (LDLs) and high-density lipoproteins (HDL). LDL is frequently referred to as “bad cholesterol,” while HDL is deemed “good cholesterol,” although it is now known that understanding cholesterol and its effects on the human body are much more complicated than this simplified breakdown. Nonetheless, it’s still very important to maintain adequate levels of the “healthy” HDL. So if you’ve been told that you need to increase your HDL levels to optimize health, keep reading to discover several HDL-boosting tips. Tip 1: Exercise! It’s true! Exercise is not just great for improving body composition, balancing blood sugar, losing weight and feeling happier, it’s also been shown to be associated with a healthier lipid and lipoprotein profile, thus lowering the risk for heart health problems.1,2 In a study comparing well-trained soccer players to sedentary controls, researchers found the average HDL-C levels in the athletes was 12.5% higher than in the nonexercising controls.2 This is important when considering that HDL can be compared to a “dump truck” for the cell; it gathers excess cholesterol left in the artery walls and carries it to the liver for disposal (breakdown and excretion).3 Too little HDL can lead to excess plaque buildup and subsequent risk of cardiovascular issues.2 One medical organization notes that benefits of exercise can be seen in as little as 60 minutes of moderate-intensity aerobic exercise per week, while a meta-analysis that included 25 studies indicates it may be closer to 120 minutes of weekly exercise to experience an HDL benefit.4,5 This meta-analysis concluded that it was the exercise duration rather than intensity or frequency that resulted in best benefits.5 And, in fact, it seems that more is better: “Each 10-minute increase in exercise duration corresponded to an approximately 1.4-mg/dL net increase in HDL-C level.”5 Tip 2: Take targeted nutritional supplements Nutraceuticals have become an indispensable and regular part of supplementing healthy living these days. Known for their vast array of health benefits, omega-3 fatty acids have been shown to also support healthy HDL levels.6 Omega-3s have two broad categories: eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), both downstream metabolites of the well-known class of anti-inflammatory promoting omega-3s called alpha-linolenic acid or ALA.7 Studies indicate it is the DHA that is most effective in improving HDL levels with one study showing a 4.49 mg/dL increase in HDL when subjects were given DHA compared to placebo subjects not given DHA.6 Tip 3: Stop smoking It’s been well-established that smoking is detrimental to health and, in particular, is a significant risk factor for cardiovascular health problems.8,9 Smoking has been shown to reduce levels of HDL and other underlying causes that lead to an increased risk of heart issues.9 The good news? When someone quits smoking, a reversal in HDL levels can be seen with the recently smoke-free person’s cardiovascular risk soon approaching that of the long-term nonsmoker.10,11 In summary, higher serum levels of HDL are positively associated with a lowered risk for the development of cardiovascular disease.12 On the flip side, a suboptimal level of HDL may increase an individual’s risk for cardiovascular issues, in both men and women.13 Keeping HDL levels within their optimal range is important. Here are three things you can do right away to ensure your HDL levels are moving in the right direction:
References:
Did you know your hormones affect your urinary tract and vaginal health?* Before menopause, your hormones rise and fall with your menstrual cycle, causing you to be more likely to develop an imbalance in your vaginal microbiome in the two weeks following the beginning of your period.1 Additionally, the hormonal shifts that come with perimenopause and menopause also affect urinary tract comfort.2 As well, disruption to your hormone levels may also cause a range of symptoms like PMS;3 heavy or light periods;3 tender or swollen breasts;4 weight gain around the butt, hips, waist, and back of arms;5-7 low mood; anxiousness; fatigue;7 and reduced libido.7 Here are some foods to eat and others to avoid to help keep your hormones in harmony: Another way to ensure you’re getting the nutrients you need to support your health is to add supplements like Wellness Essentials® Women or Wellness Essentials Women’s Prime to your daily regimen.
For additional urinary tract and vaginal health, consider taking a probiotic specific to urinary tract and vaginal health.* References
By Monazza Ahmad, B.Pharm, MSc To ensure best health outcomes in a healthcare setting, it is important to establish open, effective, and respectful communication between a patient and a healthcare practitioner. As a patient, it is not only your right but in your best interest to ask your doctor for clarification or further explanation on your health condition and treatment plan. As a healthcare provider, it is your responsibility to provide a safe and comfortable environment for your patients to openly discuss their health concerns and make sure your diagnosis and treatment plan is well understood. That said, menopause can trigger many complex and confusing symptoms that can be uncomfortable to talk about, which can make managing the symptoms difficult. When should I talk to my doctor about menopause?1 First of all, don’t feel awkward. Menopause is a natural phase of life, and your doctor is likely familiar with all possible symptoms. Describe your condition and symptoms in detail and explain how they are affecting your lifestyle and relationships. The provider needs to rule out any other underlying conditions before determining it is menopause. Reach out to your doctor if:
Don’t forget to tell your doctor if you have any allergies or other health conditions. Also mention any medications or supplements you’re already taking for menopause symptoms or other conditions. What shall I ask my doctor? Medical appointments can be stressful, and we often feel rushed. When you have a delicate issue to bring up, being prepared can help. Here are some of the questions you can ask your practitioner:
How can healthcare providers ensure the right care during menopause? Often, the reluctance from healthcare professionals to address these female issues can also cause lack of awareness. It is the healthcare provider’s obligation to make sure patients feel comfortable in discussing their menopausal concerns and understand the treatment plan.2 According to a survey by NIH, patients are likely to discuss menopause transition with providers who don’t make them feel rushed, are good listeners, and have expertise in this area of women’s health. Here are some ways to help your patients receive the best care:
What to ask your patients:3
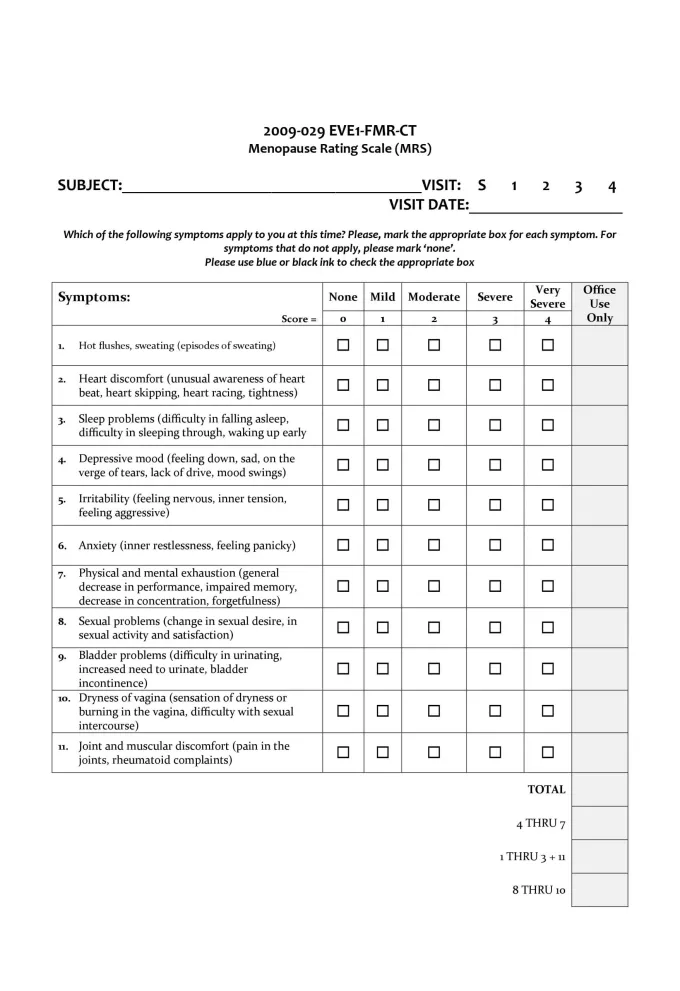
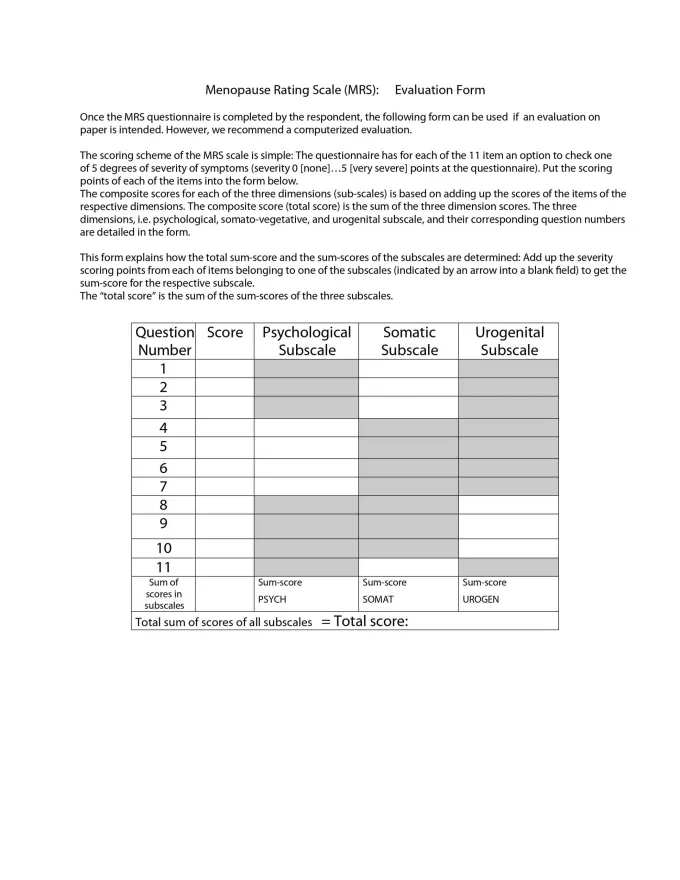
The menopausal rating scale and symptoms evaluation form can be used to track symptom improvement to see what treatment options are working:4,5 Menopause symptoms evaluation form and rating scale (click to enlarge)Menopause is a natural phase in a woman’s life, and its challenges need to be accepted and understood at work, at home, and on a social as well as a societal level. Unless these concerns are progressively communicated in a healthcare setting, it will be tough for us to understand the impact of this condition on a woman’s life.
References:
Glyphosate, first registered for use in the United States in 1974, is an herbicide widely used to kill broadleaf weeds and grasses and regulate the growth of certain plants.1,2 It is employed in agriculture, forestry, in lawn and garden maintenance, and for weed control in industrial areas.1
The use of glyphosate has skyrocketed since 1997, after crops genetically modified to tolerate glyphosate were first introduced.3 The broad usage has led to questions of whether glyphosate remains present in the food we eat and water we drink, as well as whether it is safe for humans to consume. Because glyphosate binds so tightly to soil particles after use, it is believed to be prevented from entering groundwater.4 However, this may not be the case. During a 2002 study of waterways in nine Midwestern states, glyphosate was detected in 36% of the 154 samples taken, although the highest measured concentration was still well below the maximum contaminant level (set by the Environmental Protection Agency) of 700 micrograms per liter.3 And in one study of an agricultural community in Mexico, glyphosate was detected in both groundwater and bottled water.5 Due to its lack of quick degradation in plants, glyphosate residue could be present in the food supply.4 In tests performed by researchers and consumer watch groups, glyphosate has been detected in a number of foods, including bread, honey, oat-based cereals, granolas, and snack bars.6,7 Common means of glyphosate exposure Pure glyphosate is said to be low in toxicity, but it is often mixed with other ingredients that can make the resulting product more toxic.1 Directions for applying glyphosate usually caution users to wear gloves and eye protection to avoid skin and eye irritation and to be careful not to breathe the compound to protect against nose and throat irritation.1 Although it isn’t easy for glyphosate to pass through the skin into the body, it is possible to ingest glyphosate by breathing it in while spraying or by eating or smoking after applying, without first washing your hands.1 When absorbed or ingested, most glyphosate tends to remain unchanged and leaves the body pretty quickly in urine or excrement.1 Despite that, swallowing glyphosate-containing products can result in nausea, vomiting, diarrhea, and burns in the mouth and throat.1 Intentional ingestion has resulted in fatalities in some cases.1 Because glyphosate products often contain additional chemicals, it can be hard to determine whether glyphosate alone is responsible for the adverse symptoms observed when humans come into contact with it.8 For instance, some studies suggest that the surfactant polyoxyethyleneamine used with glyphosate is more toxic than glyphosate alone.8 Agency opinions and glyphosate research Consensus on risks associated with glyphosate exposure has been tough to pin down. In April 2019, the US Environmental Protection Agency stated it “continues to find [glyphosate] poses no risk to public health when used as labeled and that it is not a carcinogen.”2 This follows a similar declaration by the European Food Safety Authority in 2015 that glyphosate is unlikely to be a carcinogen in humans.9 By contrast, the World Health Organization’s International Agency for Research on Cancer (IARC) stated in March 2015 that it had analyzed the results of over 1,000 studies and determined that glyphosate was possibly carcinogenic to humans.10 In an updated monograph released one year later, the IARC attributed the difference between its statement and the findings of other agencies to the source material, noting that most regulatory agencies reviewed nonpublic industry data from toxicological studies.10 Still, research into glyphosate’s possible carcinogenic effects continues. A recent analysis of human epidemiological studies confirmed a link between glyphosate exposure and an increased risk for non-Hodgkin’s lymphoma.11 And in another lab study, glyphosate was shown to stimulate the growth of a certain type of hormone-dependent breast cancer in cells by acting on estrogen receptors, indicating it may be an endocrine disruptor.12 Researchers are also investigating glyphosate’s impact on the human reproductive system. In one study, exposure to glyphosate-based herbicides below the toxicity threshold decreased the activity of aromatase, a key enzyme in balancing sex hormones.13 Another study in prepubertal male rats showed a decrease in testosterone levels in those given soy milk supplemented with glyphosate.14 And results of a study in pregnant mice indicate that glyphosate can cause the ovaries to fail and interfere with secretion of hormones.15 There have been cases of accidental exposure to concentrated glyphosate solutions causing neurological lesions, suggesting glyphosate could be neurotoxic in high doses.16 In a model of the blood-brain barrier, researchers observed that a high dose of glyphosate resulted in neurological damage and altered metabolism of glucose.16 Glyphosate and antibiotic resistance Recent research has uncovered a worrisome link between glyphosate use and the growing problem of antibiotic resistance.17,18 It is believed the use of glyphosate is leading to changes in microbiome composition and, as a result, increases in resistance to critical antibiotics.17 Researchers note that when bacteria are exposed to non-antibiotic chemicals such as herbicides, they can be inclined to develop resistance to antibiotics more rapidly: in some cases, 100,000 times faster.18 Looking to avoid glyphosate? The Detox Project offers Glyphosate Residue Free and Gold Standard Detox certification to bring a new era of transparency to the food and supplement industries. References:
|
Categories
All
Archives
April 2024
|
|
Join Our Community
|
|
Amipro Disclaimer:
Certain persons, considered experts, may disagree with one or more of the foregoing statements, but the same are deemed, nevertheless, to be based on sound and reliable authority. No such statements shall be construed as a claim or representation as to Metagenics products, that they are offered for the diagnosis, cure, mitigation, treatment or prevention of any disease. PAIA Manual |



















 RSS Feed
RSS Feed