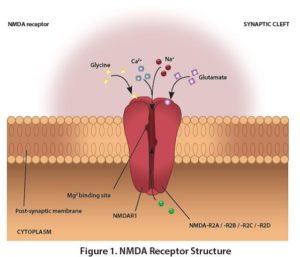
 Magnesium L-threonate is the magnesium salt of a naturally occurring vitamin C metabolite L-threonic acid. Magnesium, a divalent cation, is important for neuronal activity as it is a co-factor for enzymes present in the neurons or glial cells.1,2 Magnesium and Cognitive Health Two observational studies found that individuals with a diet rich in magnesium have a lower risk of cognitive decline:
Mechanism of Action Magnesium regulates the opening of N-methyl-D-aspartate receptor (NMDAR) in the brain. This receptor plays a critical role in cognitive function and is the target of various neurological treatments.5 Structurally, NMDAR is made up of two glycine-binding NR1 sub-units, and two of four glutamate-binding NR2 sub-units: NR2A, NR2B, NR2C, and NR2D (Figure 1). Research Highlights
Long-Term Potentiation Out of the four NR2 subunits, NR2B is of prime importance because it confers greater synaptic plasticity which helps to create and retain memories. However, the number of NR2B sub-units have been shown to decrease with age in animals.6 Overexpression of the NR2B sub-unit enhanced memory in transgenic rats and mice compared to wild-type littermates.7 NR2B is also thought to influence memory formation by increasing the long-term potentiation (LTP) through the activation of calcium/calmodulin dependent protein kinase II (CaMKII) (Figure 2).8 Long-term potentiation is long lasting increase in synaptic efficacy, which is critical for learning and memory.9 Magnesium L-Threonate Enhances Spatial Memory in Animals Magnesium L-threonate up regulated the expression of NR2B subunit in cultured hippocampal neurons.10 Compared to control, rats treated with magnesium L-threonate had:
This increase in NR2B sub-unit expression and magnitude of LTP by magnesium L-threonate translates into enhanced hippocampus dependent memory. In this study, spatial working memory, memory regarding one’s environment, and spatial orientation, were assessed at day 0 and day 24 by T maze. At day 0, rats in both groups made 30% fewer correct choices, but at day 24 aged rats treated with magnesium L-threonate made about 15% more correct choices than untreated rats (p<0.05). Interestingly, the improvement in spatial memory of aged rats declined within 12 days of stopping the treatment but improved when the treatment was re-initiated. Magnesium L-Threonate Improves Memory in Older Adults The effect of magnesium L-threonate on memory was studied in a randomized double-blind placebo controlled study with 50 men and women between 50-70 years of age with self-reported complaints of memory and concentration. Subjects were treated with 1.5-2 g/day of magnesium L-threonate, along with 200 IU of vitamin D and 30 mg of vitamin C for 12 weeks. Working memory and capacity to store and process information, measured by digit span test, improved by 13.1% at week 6 compared to placebo (p=0.023). However, this effect on working memory approached significance at week 12, which was the end of the study (p=0.064).12 Conclusion Pre-clinical studies demonstrate that magnesium L-threonate may increase synaptic plasticity through increasing the expression of one of the NMDA receptor sub-units. In vivo and clinical study results show that magnesium L-threonate positively influences cognitive measures of memory. More clinical studies are underway to further evaluate effects of magnesium L-threonate on memory and other dimensions of cognition.
1 Comment
by Ashley Jordan Ferira, PhD, RDN
Recent research from three well-known cohorts, The Nurses’ Health Study (NHS), NHS2 and Health Professionals’ Follow-Up Study (HPFS), reveals that higher magnesium intake is associated with lower risk of type 2 diabetes (T2D), particularly in diets with poor carbohydrate quality.1 Green leafy vegetables, unrefined whole grains, and nuts are richest in magnesium, while meats and milk contain a moderate amount.2 Refined foods, like carbohydrates (carb), are poor sources of magnesium. Diets with poor carb quality are characterized by higher glycemic index (GI), higher glycemic load (GL), and lower fiber intake. These poor carbs require a higher insulin demand. The typical American diet is low in vegetables and whole grains, resulting in reduced magnesium intake. The Recommended Daily Allowance (RDA) for magnesium is 310-320 mg/day for adult women and 400-420 mg/day for adult men.3 Half of the US population fails to meet their daily magnesium needs, and hypomagnesemia exists in 1/3 of adults.4-5 Magnesium is needed for normal insulin signaling; current research has linked insufficient magnesium intake to prediabetes, insulin resistance and T2D.4 Increased magnesium intake has been inversely associated with T2D risk in observational studies.6 Collaborators from Tufts University, Harvard University, and Brigham and Women’s Hospital, sought to investigate the impact of magnesium intake, from both dietary and supplemental sources, on the risk of developing T2D in subjects who had diets with poor carb quality and raised GI, GL, or low fiber intake.1 They followed three large prospective cohorts, NHS, NHS2 and HPFS (totaling over 202,700 participants). Dietary intake was quantified by validated food frequency questionnaires (FFQ) every 4 years, and T2D cases were captured via questionnaires. Over 28 years of follow-up, there were 17,130 cases of T2D. Major study findings included:1
Similar to the US population estimates, 40-50% of study participants had inadequate magnesium intake. A healthful, varied diet and supplemental magnesium (especially in diets that restrict or exclude carbohydrates, dairy or meat) are essential to ensure sufficient daily magnesium intake. Why is this Clinically Relevant?
Citations
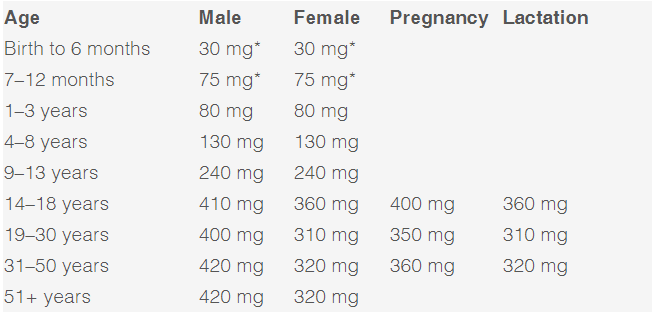
by Bianca Garilli, ND Magnesium is the 4th most abundant mineral in the human body following calcium, sodium, and potassium. Intracellularly, magnesium is the 2nd most abundant cation behind only potassium.1 The number of essential roles magnesium plays in the body is extraordinary, with over 300 enzymes requiring magnesium as a co-factor for proper functioning.1 This essential element is involved in numerous critical physiological processes such as energy production (ATP metabolism, oxidative phosphorylation, and glycolysis), protein synthesis, muscle contraction, nerve function, blood glucose control, hormone receptor binding, blood pressure regulation, trans membrane ion flux, gating of calcium channels, cardiac excitability, and synthesis of nucleic acids (RNA and DNA).1 Unfortunately, magnesium is one of the most prevalent nutrient gaps in the US. The 2015 Dietary Guidelines Advisory Committee noted a substandard intake of magnesium as compared to the Estimated Average Requirement (EAR), which is the Dietary Reference Intake (DRI) used to assess population sufficiency vs. insufficiency for nutrients.2-3 A 2016 publication in Advanced Nutrition concluded, “Approximately 50% of Americans consume less than the EAR for magnesium, and some age groups consume substantially less”.4 This is especially concerning when one considers the critical implications of long-term, frequently unrecognized magnesium deficiencies. Deficiencies in magnesium can present with overt clinical manifestations such as nausea, vomiting, lethargy, weakness, personality changes, tetany and tremor, seizures, arrhythmia, and muscle fasciculations.5 In other cases, sub clinical deficiencies may be more difficult to recognize yet have equally serious effects if left untreated. Health concerns and disease processes resulting from an underlying, subclinical magnesium deficiency may contribute to low bone mineral density and cardio-metabolic implications such as metabolic syndrome, hypertension, arrhythmia, arterial calcification, atherosclerosis, heart failure, and increased risk for thrombosis.6 A sub clinical magnesium deficiency can also disrupt sleep and cause muscle cramping, two common symptoms often glossed over but which can be signs of a bigger problem if left untreated. The impact of magnesium on these two clinical manifestations will be explored further: Magnesium and sleep A double-blind randomized clinical trial composed of 43 elderly participants between 60-75 years of age with diagnosed insomnia was conducted.7 The experimental group was given 500 mg/day of elemental magnesium for 8 weeks (250 mg elemental magnesium from 414 mg of Mg oxide, twice daily), while the control group received a placebo for the same length of time.7 A statistically significant increase was seen in sleep time, sleep efficiency, and concentration of serum renin and melatonin, as well as a significant decrease in insomnia severity index (ISI) score, sleep onset latency, and serum cortisol level.7 For many individuals, sleep is disrupted by restless leg syndrome (RLS) or periodic limb movements (PLMS).8 A study supplementing 12.4mmol of oral magnesium in the evenings for 4-6 weeks found that the overall sleep efficiency improved from 75 to 85%.9 The Mg-supplemented group also experienced a significant reduction in PLMS associated with arousal (7 PLMS/hr vs. 17 PLMS/hr at baseline).9 Magnesium and muscle cramps Muscle cramping is a common occurrence among women during pregnancy, in athletes, and in the elderly, for which magnesium is often recommended.10 There are only a few studies, however, that have reviewed the efficacy of magnesium for muscle cramping.10 In a Cochrane review, 7 trials (5 parallel, 2 cross-over design) were included, with 3 of these trials studying pregnancy-associated leg cramps in 202 females and 4 trials looking at idiopathic leg cramps in 322 participants.10 Results from the studies noted no significant improvement of muscle cramping in older adults, while results in pregnancy were mixed leading the authors to recommend further studies in this population.10 The authors of a review article in Scientifica note that the mixed findings may be explained by the potential that, “deficiencies of other elemental nutrients including calcium and potassium have also been implicated in muscle cramps and spasms. It may be that magnesium is potentially helpful in situations of magnesium deficiency but is not of use if the problem is related to deficiency of another nutrient.”1 Magnesium: Daily needs and sources Magnesium is an essential macro-mineral required by the human body. The prevalence of deficiency from serum measurements ranges from 12.5-20% of the population.11 Due to the necessity of this cation for over 300 reactions in the human body and the high risk of deficiency, magnesium levels should be routinely monitored either through blood testing and/or a diet diary review. If found to be low, magnesium stores can be replaced through increasing daily intake of the mineral through nutrition as well as routine supplementation. Foods groups high in magnesium content include green leafy vegetables, legumes, nuts, seeds, and whole grains.12 Specific foods with high magnesium levels include spinach, Swiss chard, beet greens, turnip greens, pumpkin seeds, summer squash, soybeans, sesame seeds, quinoa, black beans, cashews, sunflower seeds, brown rice and pinto beans.12 The Recommended Dietary Allowance (RDA) for magnesium varies by age, sex, and whether pregnant or lactating:13 *RDA not able to be determined; Adequate Intake (AI) reported
Supplementation with high-quality magnesium is another, targeted way to reach optimal levels and fill dietary gaps. Supplementation dosing and form can be personalized and taken orally via capsules, tablets, liquid, and even powder. Some of the different forms available in the market include Mg oxide, gluconate, chloride, citrate, sulfate, glycinate, and L-threonate. REFERENCES
Bianca Garilli, ND Dr. Garilli is a former US Marine turned Naturopathic Doctor (ND). She works in private practice in Northern California as well as running a consulting company working with leaders in the natural and functional medicine world such as the Institute for Functional Medicine and Metagenics. She is passionate about optimizing health and wellness in individuals, families, companies and communities- one lifestyle change at a time. Dr. Garilli has been on staff at the University of California Irvine, Susan Samueli Center for Integrative Medicine and is faculty at Hawthorn University. She is the creator of the Veterans for Health Initiative and is the current President of the Children’s Heart Foundation, CA Chapter. Broad health implications for general population, too by Bianca Garilli, ND, USMC Veteran
Energy drinks have become a common sight in today’s fast-paced, “get the job done” world. In fact, according to the National Institutes of Health (NIH), next to multivitamins, energy drinks are currently the most popular dietary supplement consumed by American teens and young adults.1 These drinks are marketed as a means to improve energy, stamina, athletic performance, and concentration, as well as reducing fatigue.1-3 Energy drinks are sometime considered “functional beverages” while also falling under the umbrella of drinks or dietary supplements.1,3 The main ingredient in energy drinks is caffeine at levels 70-240 mg in a 475 ml drink or 113-200 mg if consumed as an energy “shot”.1 For comparison, a 355 ml can of soda contains around 35 mg of caffeine, while an 235 ml cup of coffee provides approximately 100 mg.1 In addition to caffeine, many energy drinks also contain other ingredients which might include: B vitamins, various forms of sugar, guarana, taurine, ginseng, glucuronolactone, yohimbe, carnitine, and bitter orange; it is important to note that some of these ingredients further increase the quantity of caffeine in the energy drink, while others may substantially spike blood glucose levels.1 There are currently no regulations in place requiring the amount of caffeine to be printed on energy drink labels.1 Sales of energy drinks have skyrocketed dramatically in the past years increasing to $9.7 billion in sales in 2015 in the US alone.3 Consumption of energy drinks may have negative health consequences, including higher levels of risk-seeking behaviors, poor mental health outcomes, adverse cardiovascular effects, and heightened risk for metabolic, renal, and dental conditions.3 With marketing campaigns geared towards teenagers and the young adult population, it’s not surprising to find that males between the ages of 18-34 are the highest consumers of these drinks.1 Moreover, one-third of teenagers consume energy drinks regularly, while 51% of college students report their consumption at least once per month.1,3 Energy drinks have a strong appeal to military service members as well, particularly with the drinks’ promises of reduced fatigue and enhanced mental and physical performance. Due to the specialized nature of their work, military personnel often seek ways to:
Statistics estimate that energy drink consumption by military personnel mirrors or exceeds that of the general public, yet the health consequences of long-term, high energy drink use in military troops has not been adequately researched.2 A recent study aimed to learn more about the associations between energy drink consumption and health outcomes in military personnel post-deployment.2 In particular, mental health variables including sleep problems, depression, anxiety, PTSD, alcohol misuse, aggressive behaviors, and overall fatigue were compared to the frequency and quantity of energy drink consumption in 627 male infantry Army soldiers 7 months post-combat deployment.2 Results from this study published in Military Medicine found that approximately 75% of soldiers reported consuming energy drinks with nearly 30% of those reporting at least daily use and 16.7% reporting high level consumption (≥ 2 drinks/day).2 Additionally, when compared to the low-frequency group (no consumption of energy drink or < 1 drink/week), the high-frequency group (≥ 2 drinks/day) had increased rates of sleep problems, depression, anxiety, PTSD, and alcohol misuse; they were also more likely to demonstrate aggressive behavioral characteristics.2 Similarly, the moderate-frequency group (at least once per week or 1 drink/day) showed greater depressive symptoms when compared to the low-frequency group.2 Although energy drinks are marketed to consumers as a means to reduce fatigue, the results from this study demonstrated that moderate and high energy drink users experienced heightened fatigued when compared to low or no use.2 The high use of energy drink may, in fact, be a hindrance to both the mission objectives and troop welfare. Authors of this study conclude that, “future research should examine whether energy drink use results in greater fatigue over time.”2 Realizing that mental health, aggressive behaviors, and PTSD are potential concerns among military personnel, the high prevalence of energy drink use in this population should be reviewed. Revised guidelines from military healthcare leaders on the consumption of energy drink would be prudent to support safe and appropriate utilization of these drinks within the military. Why is this Clinically Relevant?
Link to article Citations
You try to eat well to feel good and stay healthy. While it’s optimal to get your daily nutritional needs from the foods you eat, it’s just plain difficult. There is conflicting information out there on the benefits of supplements, but the Dietary Guidelines for Americans 2015-2020 say that supplements may be useful for providing the nutrients you may be lacking from diet alone.
Still on the fence? Consider these top five reasons to add a multivitamin to your daily regimen. 1. Healthy aging. As we get older, our bodies have a harder time absorbing nutrients from food. The National Institute on Aging notes that starting around age 50, people begin to require increased amounts of certain vitamins and minerals.1,2 In fact, according to a study published in the American Journal of Clinical Nutrition, researchers found that taking a daily multivitamin & mineral supplement may help improve micro nutrient deficiencies associated with aging.3 2. Making up for eliminated food groups. While some people have to cut certain foods like nuts or gluten out of their diets due to allergies, many eliminate particular foods or food groups from their diet voluntarily. This can cause vitamin insufficiency and deficiencies that would be helped with a multivitamin. Trying a paleo diet? You might risk a shortage of calcium or vitamin D by eliminating dairy or grains. Cutting back on red meat? A multivitamin will replace the iron and B12 you would normally get from diet. 3. Getting the RDAs you’re not getting from food. You’ve probably heard that the typical Western diet doesn’t include nearly enough fruits, vegetables, whole grains, legumes, or lean protein. Because of that, you don’t always reap the vitamin and mineral benefits that those foods naturally supply. Consequently, nationally US adults are routinely failing to meet their daily requirements for vitamin A, C, D, E, and K, as well as for calcium, magnesium and potassium from diet alone, and this is including fortified sources!4 Supplementing with a multivitamin is therefore a prudent way to strategically fill those gaps on a daily basis. After all, the goal should not simply be to avoid blatant vitamin deficiencies, like scurvy with vitamin C deficiency. Borderline vitamin and mineral insufficiency are just as important to avoid and address. Even the most health-conscious eater will benefit from multivitamin support to achieve micro-nutrient sufficiency across the board. 4. Getting that extra energy to get through the day. In today’s “go-go-go” society, one of the top complaints is a general lack of energy. Instead of reaching for that third cup of coffee, remember that your cells require certain vitamins and minerals to power your busy life; especially if you’re not getting a full eight hours of sleep or eating a balanced diet, a multivitamin can help provide the nutrients you need to feel energetic throughout the day.5 5. Managing stress. Daily life stressing you out? You’re not alone. But vitamins and minerals play significant biochemical roles in supporting and preserving your brain’s cognitive processes, and studies have shown that a daily multivitamin—particularly one with high doses of B vitamins—can help to reduce stress and support a healthy mood.6 Ready to add a daily multivitamin to your diet? Be sure to check with your healthcare practitioner to see if he or she has personalized recommendations for you and to ensure that any medications you’re currently on won’t interfere with their effectiveness or the effectiveness of the multivitamin ingredients. Citations
by Bianca Garilli, ND
In a single year, Clostridium difficile (C. difficile) is responsible for nearly 500,000 infections among patients in the US. Approximately 29,000 of these patients die within 30 days of initial diagnosis of the infection, and 15,000 of those deaths can be attributed directly to C. difficile infection (CDI). C. difficile is now the most common microbial cause of healthcare-associated infection in US hospitals, with excess health care costs in acute care facilities estimated to be $4.8 billion per year.1 C. difficile, an anaerobic toxin-producing gram-positive spore-forming bacterium, is also the leading cause of nosocomial diarrhea worldwide, with recent data indicating an increase in both the incidence and severity of infections.2 The most common cause of CDI is antibiotic exposure. The use of antibiotics alters the balance of flora in the gastrointestinal tract, allowing for the seeding and growth of C. diff, whose transmission is via the fecal-oral route. The manifestation of CDI is typically seen while individuals are still on antibiotic therapy and within the first month after commencing antibiotics. However, the risk of CDI may continue for up to 90 days after antibiotic treatment. Antibiotics that have been shown to carry the highest risk for CDI prevalence include clindamycin, cephalosporins and fluoroquinolones.2 Interestingly if not ironically, the recommended therapy for milder cases of CDI, including for initial and second recurrences, is antibiotics. More recently, fecal transplantation has emerged as a highly effective therapy for recurrent CDI.2 With a clear understanding of root cause and effects of CDI, altering the factors leading up to CDI becomes critical for reducing the incidence of the infection. Two such approaches have been the focus of recent research:
Reducing antibiotic use A recent study conducted by the CDC showed that a 30 percent reduction in CDI-associated antibiotics use in hospitals could reduce CDI by more than 25% in hospitalized and recently discharged patients.1 Similar results were found in a retrospective Canadian hospital study, where a 10% decrease in overall antibiotic use yielded a 34% decrease in CDI.1 Following these compelling findings, the UK adopted changes to antibiotic prescribing which has reduced their CDI infection rate by well over 60%, clearly indicating that implementing such steps in a large population is both possible and favorable.1 Giving probiotics with antibiotics A systematic review and meta-analysis of 31 randomized controlled trials was recently published in the Cochrane Database of Systematic Reviews.3 The objective of the comprehensive review, which included a total of 8672 patients who completed the analyzed trials, aimed to assess the efficacy and safety of probiotics (any strain; any dose) as a potential preventative strategy for C. difficile-associated diarrhea (CDAD) in adults and children. The Cochrane analysis demonstrated that probiotic use resulted in a 60% reduction in the risk of CDAD. CDAD incidence in the probiotic groups was 1.5% compared to 4.0% in the control groups (placebo or no treatment). The authors of the review conclude that the evidence to date suggests that probiotics are effective for preventing CDAD and that short-term use of probiotics appears safe and effective when used along with antibiotics in patients who are not immune compromised or severely debilitated.3 Why is this Clinically Relevant?
Link to article
by Bianca Garilli, ND
Introduction You’re sitting in a relaxing chair at the bookstore with no acute stress or problems, when out of the blue your heart begins to race, flutter, and skip beats. This is not the first time this has happened and you wonder if you might be experiencing a medical emergency. Should you be concerned and call 911, or simply ignore the strange fluttering feelings and continue reading? The answer, as is often the case in medicine: “It depends”. Most likely, though, you are experiencing a common occurrence known as a heart palpitation.1 Heart palpitations can be felt not just in the chest, but often in the throat and neck as well. They may occur during times of activity or while at rest. When experienced for the first time, the flipping and fluttering feeling can be uncomfortable and frightening. However, not all episodes of heart palpitations are dangerous; therefore differentiating those requiring medical intervention from harmless episodes is important.2 What causes heart palpitations? Heart palpitations can have a myriad of causes. Some individuals will experience heart palpitations due to an underlying medical condition such as a hyperthyroidism or other thyroid disorders, blood sugar dysregulation, anemia, or from a drop in blood pressure.2 Heart palpitations may also show up alongside fever or dehydration and some women will even experience hormonally-driven heart palpitations during menstrual cycles, pregnancy, or menopause.3 In certain cases heart palpitations may, in fact, be a sign of a more emergent or progressive chronic condition as in the case of arrhythmias, a heart attack, heart failure, heart valve disease, or cardiomyopathy.2 Heart palpitations accompanied by fainting, dizziness, shortness of breath, or chest pain should be evaluated right away.3 Many times, however, heart palpitations are not indicative of a chronic illness or acute medical situation, but instead may be provoked by our emotional state such as acute stress, fear, anger, or anxiety.3 Other times, daily activities or habits like consuming too much caffeine or alcohol or using nicotine, energy drinks, or illicit drugs can trigger palpitations.3 Other culprits of palpitations include strenuous exercise, electrolyte imbalance, fatigue, and certain medications and supplements. For example, certain medications may cause heart palpitations as a side effect, including: inhaled corticosteroids and albuterol used for asthma; antibiotics such as azithromycin; over-the-counter decongestants containing pseudoephedrine or phenylephrine typically used for colds and coughs; and serotonin and norepinephrine reuptake inhibitors (SNRIs) prescribed for depression.3-4 Some supplements such as yohimbe and ephedra (which was banned in the US by the FDA in 2004) may also lead to heart palpitations.5 Hawthorn, an herb which has shown benefits in mild-to-moderate heart failure and sometimes used for managing blood pressure, has been shown to occasionally cause heart palpitations in some people depending on the dose and the individual.4,6 What to do about your heart palpitations? In order to determine the seriousness of your heart palpitations, it’s important to discuss your specific situation with your healthcare provider (HCP), who may decide to order follow-up tests such as blood work, EKG, echocardiogram, or a Holter monitor.3 Steps for reducing or eliminating heart palpitations will depend on whether the underlying cause is a specific medical condition or something more immediately modifiable. Removing or reducing intake of any palpitation-triggering substance is an obvious step in treating this issue. Are there natural approaches to reduce palpitations? Lifestyle modifications can help address palpitations caused by environmental inputs such as stress or emotional situations, nutrition, or exercise. A variety of mind/body techniques can help put a dent in the stressors of life; some examples include tai chi, qi gong, yoga, meditation, breathing, prayer, and biofeedback. Balancing electrolytes through healthful nutrition, hydration, and personalized supplementation is also often helpful. Research indicates that supplementing with CoQ10 or magnesium may provide palpitation relief in some individuals.7-8 One study investigating the effects of CoQ10 on heart failure found that 50 mg/day for 4 weeks led to a reduction in heart palpitations in participants.7 Another study looked at magnesium’s potential role in reducing symptoms associated with mitral valve prolapse in patients with low serum magnesium. Heart palpitations, along with anxiety, weakness, chest pain, and dyspnea are often seen with mitral valve prolapse. After 5 weeks of daily magnesium supplementation (600 mg/day magnesium carbonate supplement given TID the 1st week and BID in weeks 2-5), participants reported a significant reduction in all mitral valve-associated symptoms, including heart palpitations.8 In some cases, heart palpitations may be triggered by certain foods, in particular foods with monosodium glutamate (MSG), nitrates, or too much sodium. In these cases, considering an elimination diet or keeping a food journal may help determine which foods are exacerbating the condition.3 In many situations, heart palpitations are not serious and will go away on their own. Be sure to check with your HCP to discuss your individual experiences and concerns you may have. Citations
Dr. Garilli is a former US Marine turned Naturopathic Doctor (ND). She works in private practice in Northern California as well as running a consulting company working with leaders in the natural and functional medicine world such as the Institute for Functional Medicine and Metagenics. She is passionate about optimizing health and wellness in individuals, families, companies and communities- one lifestyle change at a time. Dr. Garilli has been on staff at the University of California Irvine, Susan Samueli Center for Integrative Medicine and is faculty at Hawthorn University. She is the creator of the Veterans for Health Initiative and is the current President of the Children’s Heart Foundation, CA Chapter. by Noelle Patno, PhD Colonoscopy is one of the most common medical procedures, used for screening various bowel diseases, including colon cancer, to examine for any pathological findings (e.g. polyps, ulcers, or inflamed tissue). Barriers to colonoscopy include patients’ fears related to invasiveness, pain, or complications.1 Complications may be as minor as bloating or as severe as bowel perforation (rare); factors that may predict increased risk for complication include insufficient bowel preparation (not completely cleaned out and therefore more difficult to visualize the tissue) or incomplete colonoscopy (for example, not completely scoped due to not fully cleaned intestine or tumor obstruction).2 In reality, the incidence of minor adverse events is quite low, typically occurring within the first two days after the colonoscopy and uncommon after two weeks.3 Most people go to work the day after the colonoscopy, but in some cases, feeling sleepy, weak, or significantly bloated and in abdominal pain may cause people to miss work.4 While the majority of studies related to colonoscopy evaluate new technologies to improve bowel preparation5-7 and pain during the procedure,8,9 less research focuses on the majority of minor symptoms occurring post-procedure. One of these pain-reducing techniques is carbon dioxide insufflation, (not available at all sites for colonoscopy procedures) which can reduce pain during and after colonoscopy.8-9 Experiences after colonoscopy render it less likely for patients to return for future endoscopies if they are needed,10 so more techniques to improve the post-colonoscopy experience are warranted. Since the gut microbiota is severely disrupted if not obliterated during bowel cleansing (and this dysbiosis contributes to symptoms of bloating and pain), probiotics may be a potential option to improve gastrointestinal (GI) symptoms post-colonoscopy. Recently, a combination of probiotic strains consumed post-colonoscopy was evaluated for the endpoints of bloating, abdominal pain, and altered bowel function.11 Study characteristics include:11
Major findings include:11 1) Pain reduction by ~19 hours for those consuming the NCFM + Bi-07 probiotic. Specifically, the number of pain days post-colonoscopy decreased from 2.78 to 1.99 (p=0.032). 2) Subgroup analyses demonstrated that patients with pre-existing abdominal pain receiving the probiotic intervention experienced fewer pain days (2.16 vs. 4.08, p=0.0498) 3) There were non-significant decreases in bloating (2.52 vs. 2 days, p=0.111) and time to return to normal bowel habits (3.42 to 3.05, p=0.280). The authors propose that the dual-strain probiotic supplement may have facilitated improvements via several possible mechanisms: inhibiting pathogens from binding, modulating the immune system, and improving colonic motility and transit, as well as affect the sensitivity of the intestines.11 Lactobacillus acidophilus NCFM12 and Bifidobacterium lactis Bi-07 have been isolated from human sources and have been extensively studied in preclinical and clinical studies and widely used, particularly together in RCTs demonstrating immune benefits in children13 and adults.14 NCFM and Bi-07 have been shown to aid in pain relief from bloating in a double-blind, placebo-controlled trial in patients with functional bowel disorder15 as well as in case reports of irritable bowel syndrome.16 The D’Souza et al. publication described above demonstrates another application for the NCFM and Bi-07 probiotic strain combination, beyond its immune and GI benefits, for the specific attenuation of pain post-colonoscopy.11 Citations
Noelle Patno, PhD Dr. Noelle Patno is the Nutrition Scientist for Digestive Health at Metagenics. Dr. Patno received her PhD in Molecular Metabolism and Nutrition and Masters in Translational Science from the University of Chicago, studying the role of microbial components in intestinal epithelial cell survival related to inflammatory bowel disease. Prior to her graduate studies, Dr. Patno received a chemical engineering degree from Stanford University and worked as an engineer. She has personal experience and interest in preventive nutrition and nutritional therapies for chronic disease, and her current role involves researching and developing probiotics, prebiotics, and other nutritional programs for the promotion of digestive and overall health. Multi-Strain Probiotic Improves Insulin Resistance in Patients with Diabetes | Blog | Metagenics5/11/2019 Targeted probiotic in personalized therapeutic plan for patients with diabetes shows promise by Bianca Garilli, ND and Ashley Jordan Ferira, PhD, RDN Type 2 diabetes (T2D) is no longer a Western world phenomena, but rather a global epidemic, with research revealing an association between higher T2D rates and a country’s wealth or economic growth.1 As a clear example, in a publication titled “Prevalence of type 2 diabetes in the Arab world: impact of GDP and energy consumption”, it was observed that the higher a country’s gross domestic product (GDP), the higher the T2D prevalence.1 T2D rates in these regions include Kingdom of Saudi Arabia- 31.6%, Oman- 29%, Kuwait- 25.4%, Bahrain- 25%, and United Arab Emirates- 25%.1 Recognizing the worldwide impact of T2D, it is critical to identify underlying causes and practical, implementable tools for prevention and treatment. It is well documented that T2D is a chronic, inflammatory condition. Higher levels of lipopolysaccharides (LPS) have been observed in diabetic vs. non-diabetic individuals.2 LPS are Gram-negative bacterial fragments that are considered endotoxins, and can, if left untreated, overgrow in the gastrointestinal tract leading to increased gut permeability.3 A “leaky gut” environment increases the opportunity for these endotoxins to migrate out of the gut and into the circulation, ultimately contributing to systemic inflammation.3 Probiotics have been studied in various models to determine their effects on LPS growth and proliferation and whether targeted probiotic administration aimed at mitigating LPS effects can reduce systemic inflammation, in particular in the T2D population.4-5 The limitations of previous research included short-term duration (≤3 months) and the utilization of mono-strain supplementation.3 To augment the current literature on this topic, a longer study (6 months) was conducted in a randomized, double-blind, placebo-controlled fashion to examine the impact of probiotics on endotoxemia, inflammation, and cardiometabolic disease risk in Arab patients with T2D.3 In this study, 61 Saudi adults (35 females) aged 30-60 years completed the 6-month trial: 30 in the placebo group and 31 in the probiotic group.3 The placebo and probiotic groups were randomly allocated to powder sachets, to be dissolved in a glass of water twice daily, before breakfast and bedtime. The probiotic intervention provided 2.5 billion CFU/g BID and included the following strains: Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19, and L. lactis W58.3 No additional therapeutics such as exercise or dietary recommendations were included during the course of the study in either group.3 In the probiotic group, significant changes in glycemic indices, lipid profile, inflammatory markers, endotoxin levels, and adipocytokine profile were observed at 6 months vs. baseline:3
The improvements in endotoxin load, inflammation, and cardiometabolic profile over time in the probiotics group are noteworthy, but they were not clinically significant when compared to the placebo group.3 Comparing the probiotic intervention to the placebo group: There was a significant and clinically relevant decrease in HOMA-IR (↓64.2%) in the probiotic group.3 HOMA-IR is correlated with most other cardiometabolic indices measured, so one could posit a potentially broader cardiometabolic benefit from the probiotic intervention, but this and other hypotheses should be explored in a future study with an adequately powered sample size. Why is this Clinically Relevant?
References
Targeted probiotic - a cornerstone of root-cause approach to disease management and wellness by Melissa Blake, BSc, ND The use of probiotics has grown substantially over the last several years. Propelled by development in sequencing methods and analytical techniques, there has been a significant increase in knowledge and understanding about the importance of a healthy microbiome.1 The currently accepted definition of a probiotic states they are “live microorganisms which when administered in adequate amounts confer a health benefit on the host”.2 Is this relatively broad definition sufficient for clinicians to guide treatment? Can we assume that any live organism in relevant doses will achieve positive clinical outcomes? We’ve been talking about the use of probiotics for over a hundred years,6 but when it comes to probiotic therapy, what do we really know? A brief history lesson As early as the 1680's, long before the term probiotic was coined, Antonie van Leeuwenhoek was studying his oral and fecal microbiota. He noted striking differences in these microbes, as well as in samples collected from healthy vs. unhealthy people at both of these anatomical sites.3 The notion of natural, innate immunity furthered our understanding of gut bacteria. The concept, first discussed by scientist Élie Metchnikoff and for which he was awarded a Nobel Prize in 1908, encountered much resistance from the medical community.4 Metchnikoff insisted disease was more than the germ theory and highlighted the importance of a healthy host. The health of the host, he believed, was largely dependent upon having diverse intestinal flora.5 Metchnikoff’s research suggested that a diet rich in fermented dairy products, due to high content of Lactobacilli, had a positive influence on health and longevity.6 The concept of “probios” (pro-bios, conducive to life of the host) was born. Research in gut microbiology has become a significant area of interest, including the establishment of the Human Microbiome Project (HMP) in 2008. The HMP has characterized the microbial communities found at several different sites on the human body: nasal passages, oral cavity, skin, gastrointestinal (GI) tract, and urogenital tract and has examined the role of these microbes in human health and disease.7 In 2012, the potential for a mammary microbiome was suggested and later confirmed in a study published in 2014, which identified widespread bacteria within the mammary glands, irrespective of lactation.8 The evidence continues to establish the diversity of the human microbiome, not only from one person to another, but also across specific body sites.9 The human microbiome A balanced and diverse microbiota plays a role in human health during the lifecycle. Growing evidence supports a connection between maternal microbiome and pregnancy outcomes, including preterm birth, preeclampsia, gestational hypertension, and gestational weight gain, as well as having an impact on infant health.10 Infancy is a critical time in the development of commensal gut bacteria and is influenced by pre- and postnatal exposures, including the maternal microbiome, delivery method (C-section vs. vaginal birth), diet, and medical interventions.11 Such factors can negatively or positively influence the balance of an individual’s microbiome and may impact short- and long-term health outcomes.12 Modifications to the infant gut microbiota may impact childhood obesity risk,13 atopic disease,14 as well as various GI conditions.15 After initial colonization, factors such as age, gender, diet, environment, stress, and the use of antibiotics continue to influence the microbiome. Changes in the GI and respiratory microbiome of adults have been implicated in the pathogenesis of chronic pulmonary diseases, including asthma and allergies.16 Dysbiosis has also been associated with psoriasis,17-18 psoriatic arthritis,18 and inflammatory bowel disease,19 suggesting a direct link between a balanced microbiome and the health of the GI and immune systems. Studies have also connected highly abundant levels of specific genera of bacteria with leanness and have shown they play a role in regulating blood sugar and insulin levels.20 Recent reports suggest that neuroinflammation is an important causal mechanism in cognitive decline. This inflammatory status could be triggered by changes in the gut microbiota composition.21 Evidence is connecting the dots between gut bacteria, altered intestinal permeability, and blood brain barrier integrity.22 A disruption in gut flora may, through several mechanisms, contribute to a “leaky brain”, making the brain more susceptible to circulating substances and contributing to cognitive dysfunction. Further research is warranted in this exciting area of scientific study. As clinicians, we cannot erase the past or possibly impact all the factors that influence the microbiome of our patients. However, we can partner with our patients to help them make positive lifestyle changes. Diet and targeted probiotic therapy are powerful tools. Consumption of excess saturated fats and added sugar influences the microbiota composition, which may lead to an imbalanced microbial population in the gut.22 By modifying risk factors and targeting the microbiome, Functional Medicine practitioners have an opportunity to both prevent and manage disease with individualized nutrition and probiotic therapy at any age. Evidence for an individualized approach Convincing evidence of the human health implications of probiotics exists. Hundreds of well-controlled trials, systematic reviews, and meta-analyses have helped define the appropriate use of probiotics and their valuable benefits. The evidence suggests, however, that probiotic therapy is far from a one-size-fits-all approach. In fact, studies show clearly defined benefits are associated with specific strains of bacteria. Here we discuss several that have substantial evidence to support their targeted clinical uses in specific populations: Lactobacillus acidophilus NCFM
Summary Although we have much more to learn, advancements in our understanding of the human microbiome continue to provide exciting approaches to Functional and personalized medicine. Probiotic therapy is a cornerstone of a root-cause approach to wellness and disease management. Citations
Dr. Melissa Blake is a clinical specialist on the Medical Information team at Metagenics. She completed her pre-medical studies at Dalhousie University in Halifax, Nova Scotia and obtained her naturopathic medical training from the Canadian College of Naturopathic Medicine. Dr. Blake has over 10 years of clinical experience, specializing in the integrative and functional management of chronic diseases. |
Categories
All
Archives
April 2024
|
|
Join Our Community
|
|
Amipro Disclaimer:
Certain persons, considered experts, may disagree with one or more of the foregoing statements, but the same are deemed, nevertheless, to be based on sound and reliable authority. No such statements shall be construed as a claim or representation as to Metagenics products, that they are offered for the diagnosis, cure, mitigation, treatment or prevention of any disease. |








 RSS Feed
RSS Feed